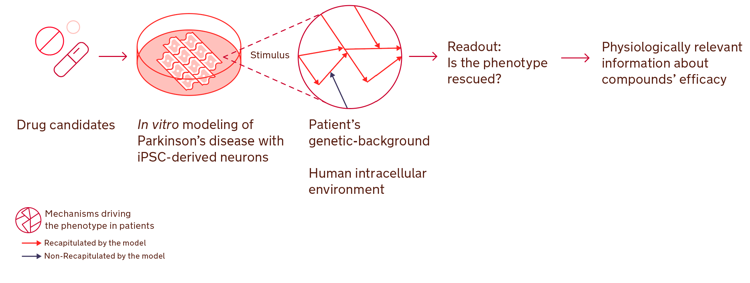
It is estimated that more than 9.4 million people in the world live with Parkinson’s disease [1] and no disease-modifying therapy has been found to date. Repurposing FDA-approved drugs can be a very time- and cost-effective strategy compared to traditional drug discovery, and has become a popular option to address the urgent therapeutic need for Parkinson’s and other diseases. Using human iPSC-based disease models with repurposing strategies can make drug discovery even more efficient, providing accurate predictions of compounds’ efficacy and reducing the need for animal testing.
The loss of dopaminergic neurons in the substantia nigra is a well-recognized hallmark of Parkinson’s disease. A contributor to this degeneration is decreased activity of the lysosomal enzyme b-glucocerebrosidase (GCase) that leads to a toxic pathogenic cascade. Therefore, increasing the activity of GCase enzyme might be a viable therapeutic approach to treat several forms of Parkinson’s disease.
In this highlighted article, Lena F. Burbulla and colleagues screened 1280 FDA-approved drugs that could potentially bind GCase and identified quetiapine as the compound with greatest affinity [2]. Then, human iPSC-derived dopaminergic neurons were used to test quetiapine impact in GCase activity in vitro and understand its capacity to rescue Parkinson’s hallmarks. The iPSC-derived models used revealed that quetiapine treatment increases GCase expression and activity in vitro, and partially rescues Parkinson’s related phenotypes in cells derived from patients with two different common genetic causes of Parkinson’s disease.
Key Takeaways
-
Human iPSCs can be differentiated into specific neuronal populations and recapitulate Parkinson’s disease-linked phenotypes to serve as relevant models for drug discovery.
-
The findings of this study highlight the effectivity of repurposing strategies for drug discovery, to address the urgent therapeutic need for Parkinson’s disease and other neurodegenerative diseases.
-
Human iPSC-derived models proved to further simplify the process of drug-repurposing, as they provide physiologically relevant information about the efficacy of therapeutic candidates faster.
References
[1] N. Maserejian, L. Vinikoor-Imler, A. Dilley. Estimation of the 2020 Global Population of Parkinson’s Disease (PD) [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/estimation-of-the-2020-global-population-of-parkinsons-disease-pd/. Accessed May 11, 2022.
[2] Burbulla, L. F., Zheng, J., Song, P., Jiang, W., Johnson, M. E., Brundin, P., & Krainc, D. (2021). Direct targeting of wild-type glucocerebrosidase by antipsychotic quetiapine improves pathogenic phenotypes in Parkinson’s disease models. JCI insight, 6(19). https://doi.org/10.1172/jci.insight.148649
