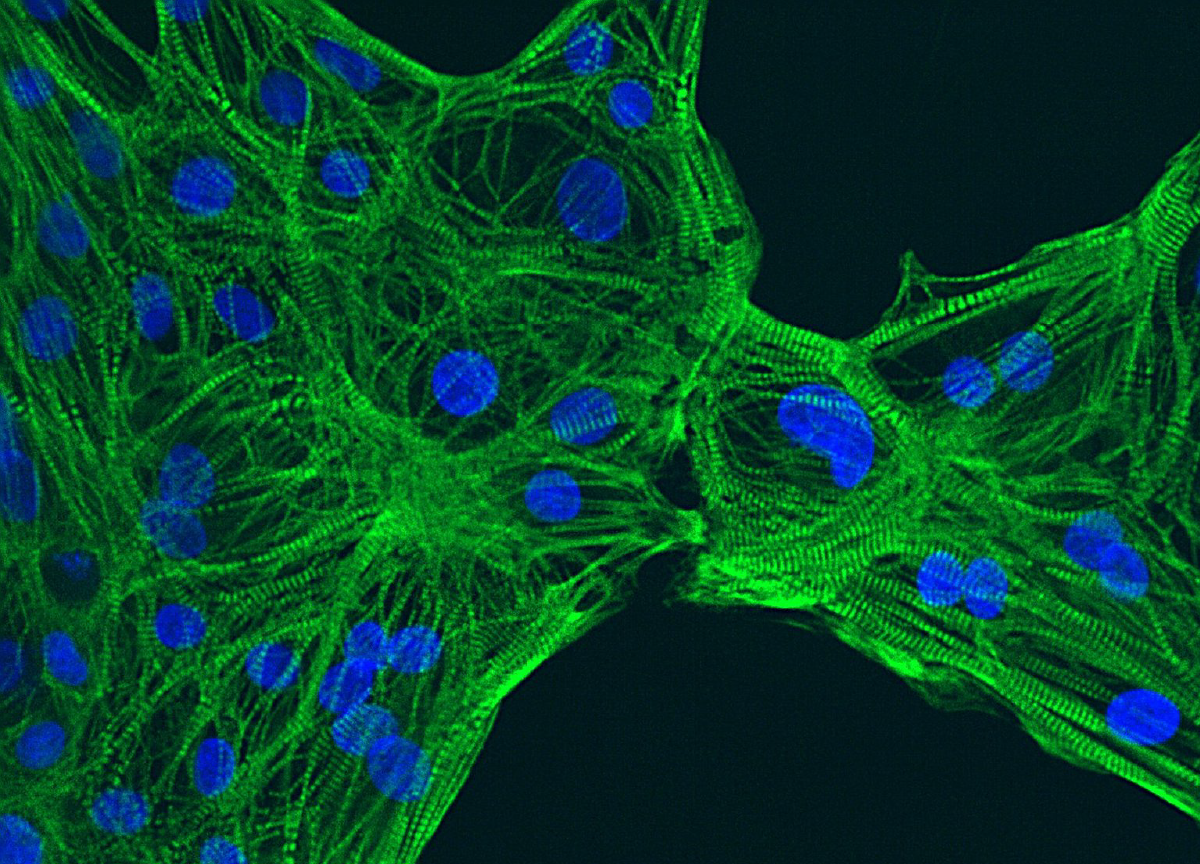
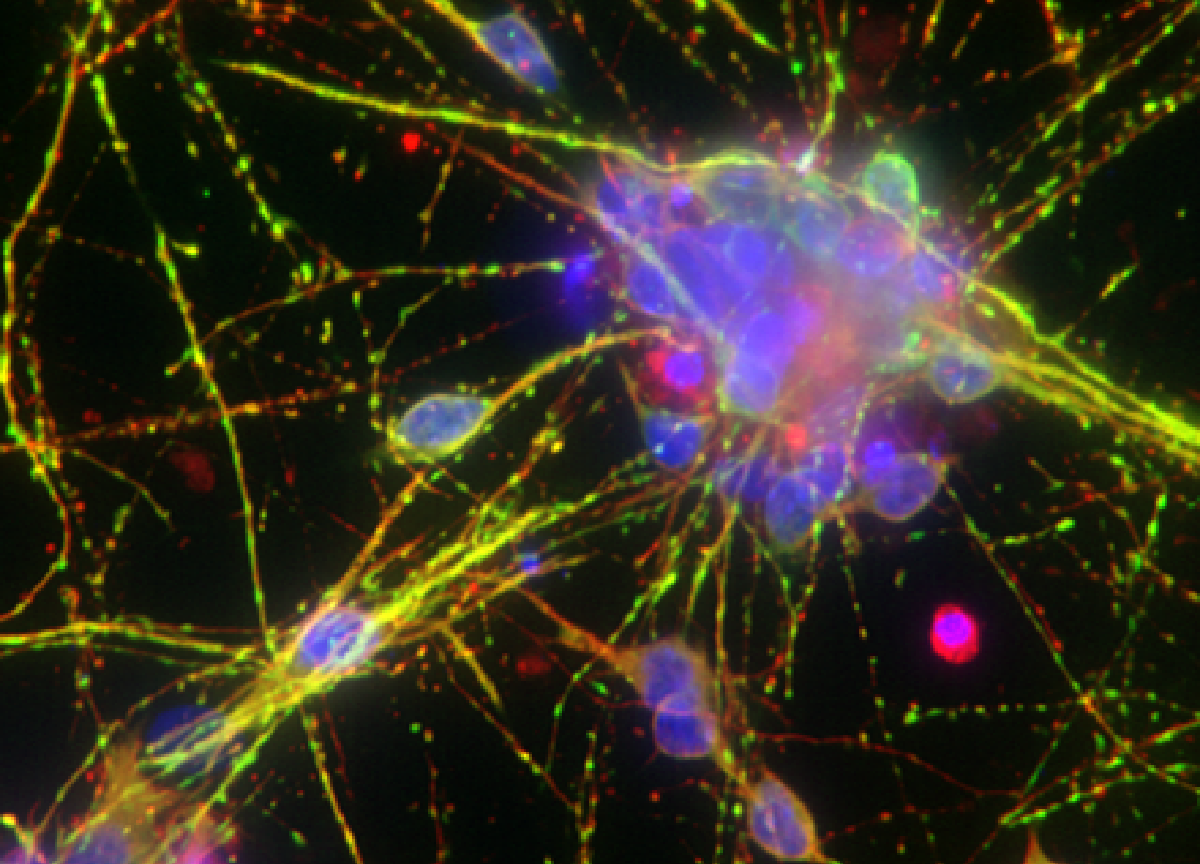
High content imaging
Phenotypic changes in cell models can provide valuable insights about the efficacy, safety and mechanism of action of therapeutic candidates. High content imaging (HCI) applies automated microscopy, fluorescent imaging and multiparameter algorithms to visualize phenotypic changes. At Ncardia, we have the expertise to combine iPSC-derived healthy and diseased models with consistent HCI assays to help you make critical decisions faster and with higher confidence.
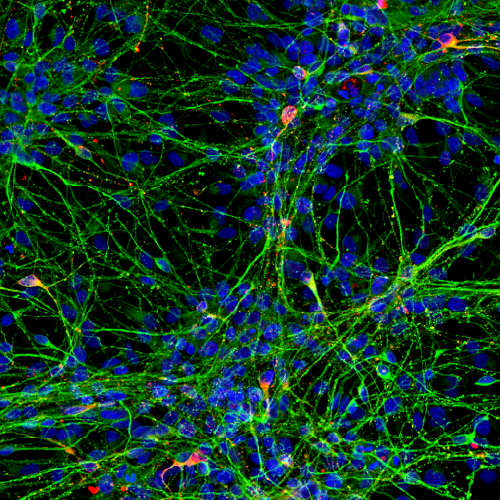
- 3D phenotypic characterization
- Assessment of multiple targets simultaneously
- Visualize complex cellular and sub-cellular structures
Do you want to explore how this assay can help you progress your drug discovery programs?
Applications
HCI based on iPSC-derived models enables the study of drug-induced morphological and spatial changes in the cells. It can be used as a primary screening for hit and lead identification or to visualize changes in more complex cellular systems during lead optimization. Our scientists optimize the assays and process the data to deliver relevant information on cell viability, proliferation, morphology or biomarker expression, among others.
High-throughput screening of anti-hypertrophy drugs based on NT-proBNP biomarker detection with high content imaging
To identify new treatments for cardiac hypertrophy, our scientists generated an iPSC-derived model of hypertrophic cardiomyopathy and used HCI for hit validation with 341 compounds. The intracellular expression of NT-proBNP biomarker was used as readout to determine the efficacy of the anti-hypertrophic hit compounds.
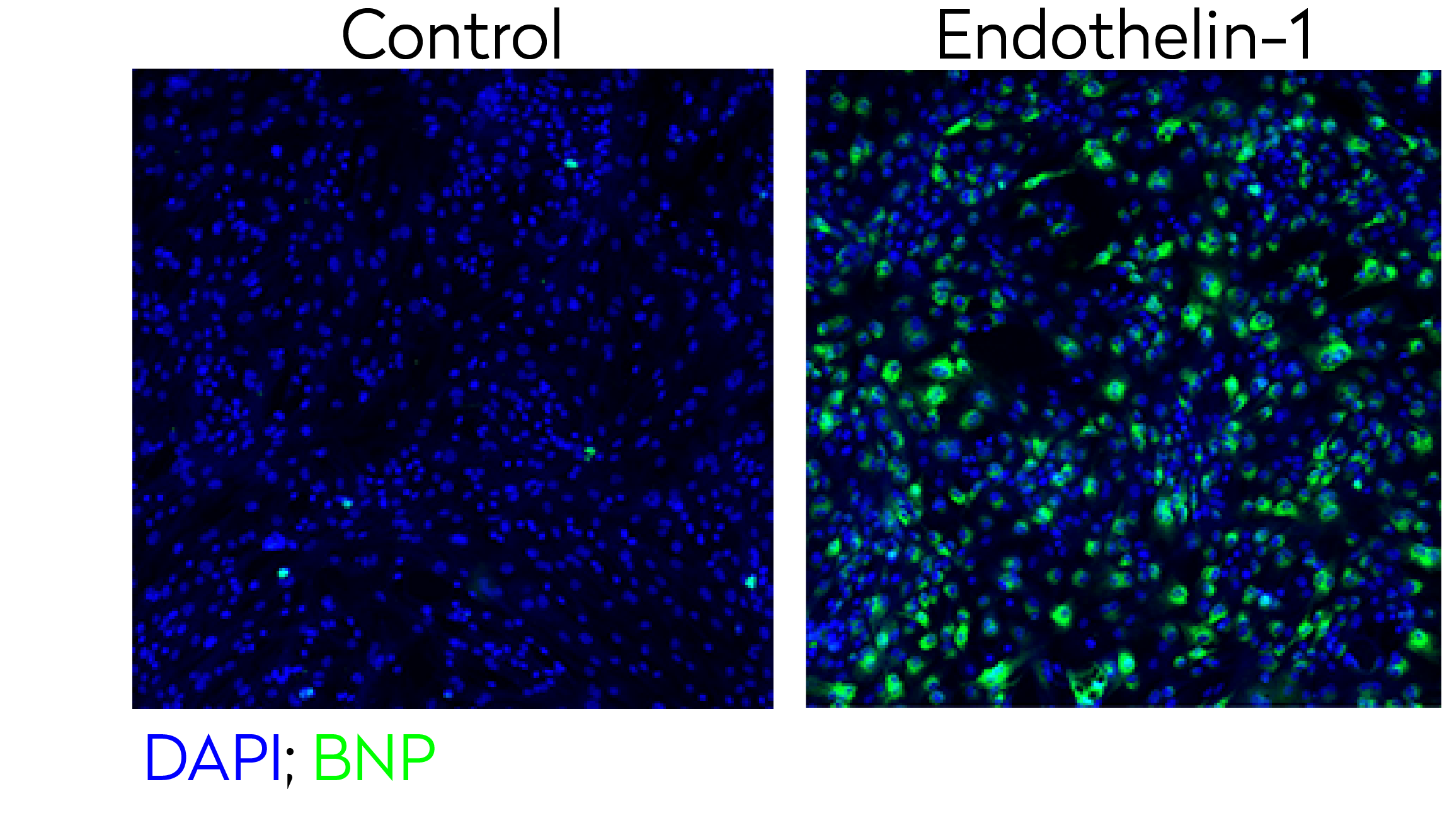 Representative confocal images of control and endothelin-1 treated human iPSC-derived cardiomyocytes stained for BNP hypertrophy biomarker (green) and DAPI (blue - nuclei).
Representative confocal images of control and endothelin-1 treated human iPSC-derived cardiomyocytes stained for BNP hypertrophy biomarker (green) and DAPI (blue - nuclei).
Evaluation of lysosomal dysfunction in a human iPSC-derived model of Alzheimer’s disease with high content imaging.
Lysosomal dysfunction is a hallmark for neurodegeneration. Using HCI, we can detect lysotracker marker to localized lysosomes and determine the capacity of new therapeutic candidates to rescue lysosomal dysfunction.
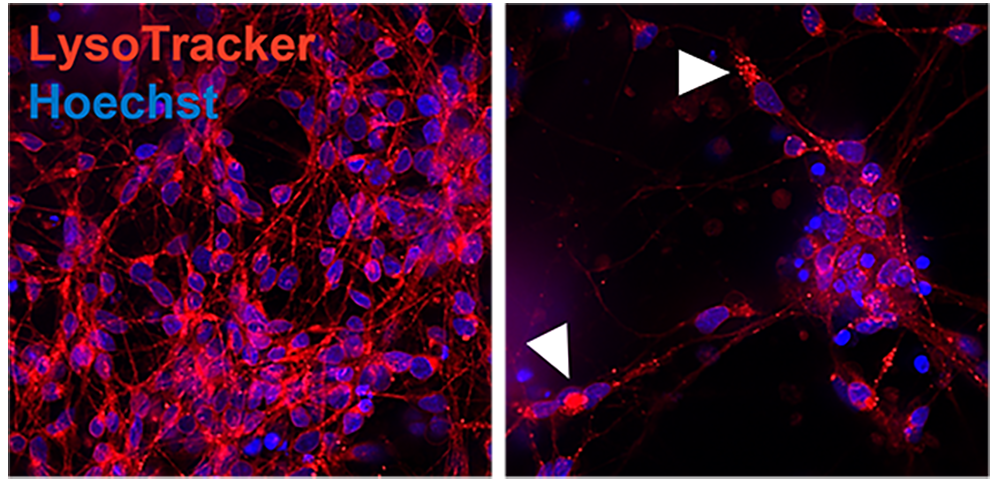 Representative confocal image of control and AD iPSC-derived neurons showing lysosomal distribution and pattern (Lysotracker, red). Arrowheads point to lysosome accumulation in the cytoplasm.
Representative confocal image of control and AD iPSC-derived neurons showing lysosomal distribution and pattern (Lysotracker, red). Arrowheads point to lysosome accumulation in the cytoplasm.
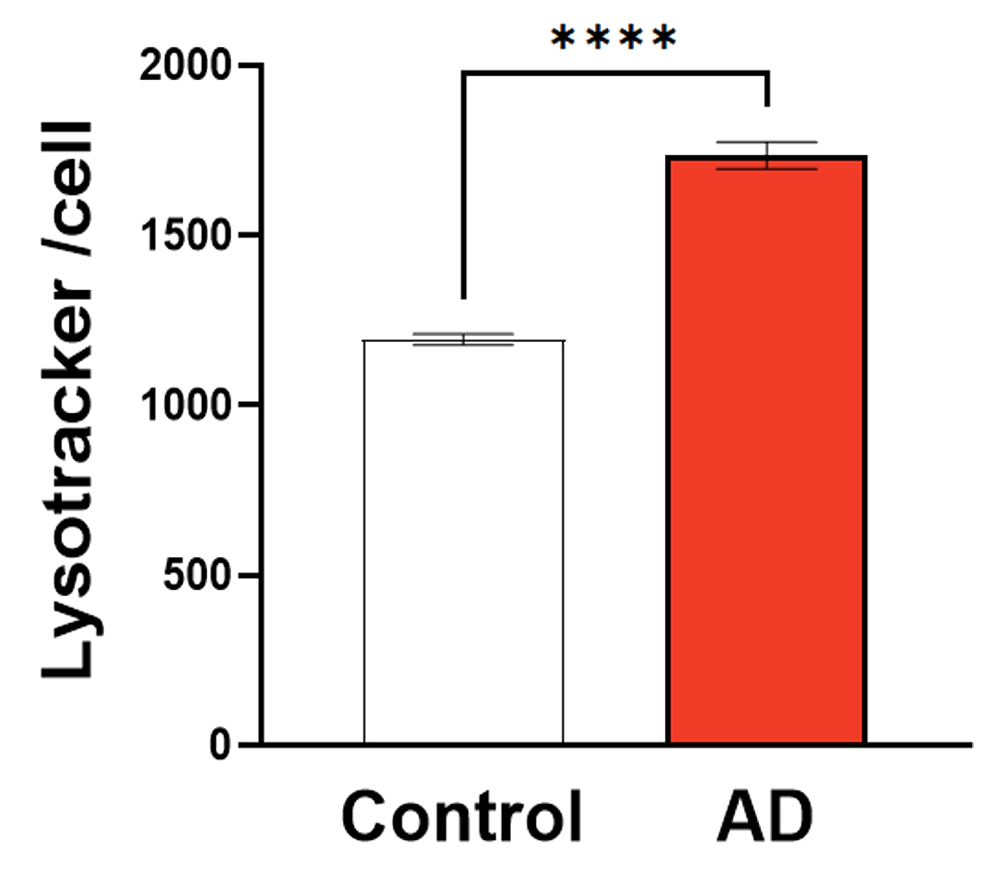 Graph showing quantification of Lysotracker cell average intensity (RFU) in control and AD iPSC-derived model
Graph showing quantification of Lysotracker cell average intensity (RFU) in control and AD iPSC-derived model
Our work centers on a simple yet powerful premise:
When we combine deep iPSC knowledge, broad assay capabilities and a demonstrated ability to integrate the biology of human diseases into preclinical research, we can help drug developers make critical decisions earlier and with more confidence.
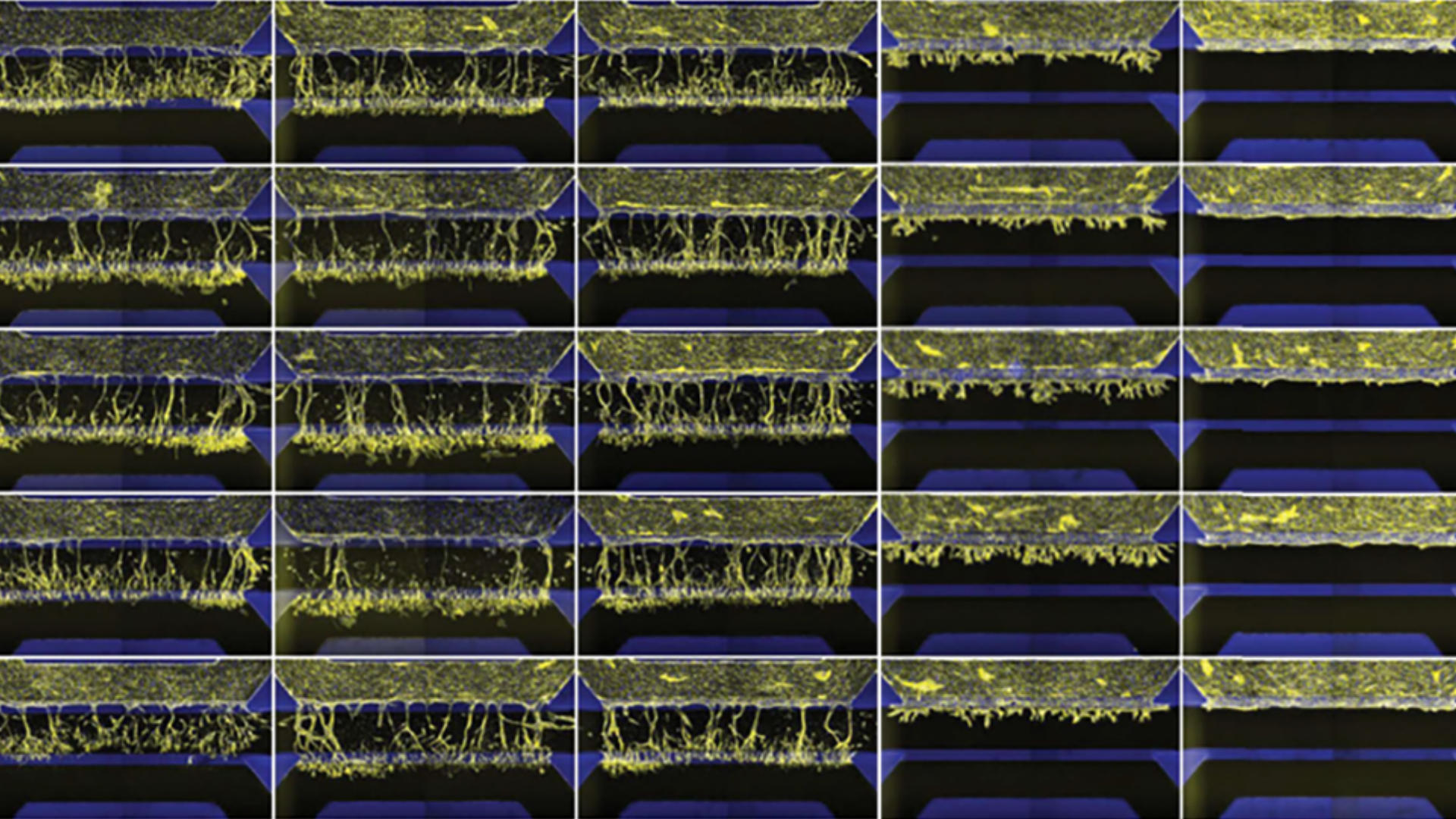
Study compound-induced effects on blood vessel formation
Assay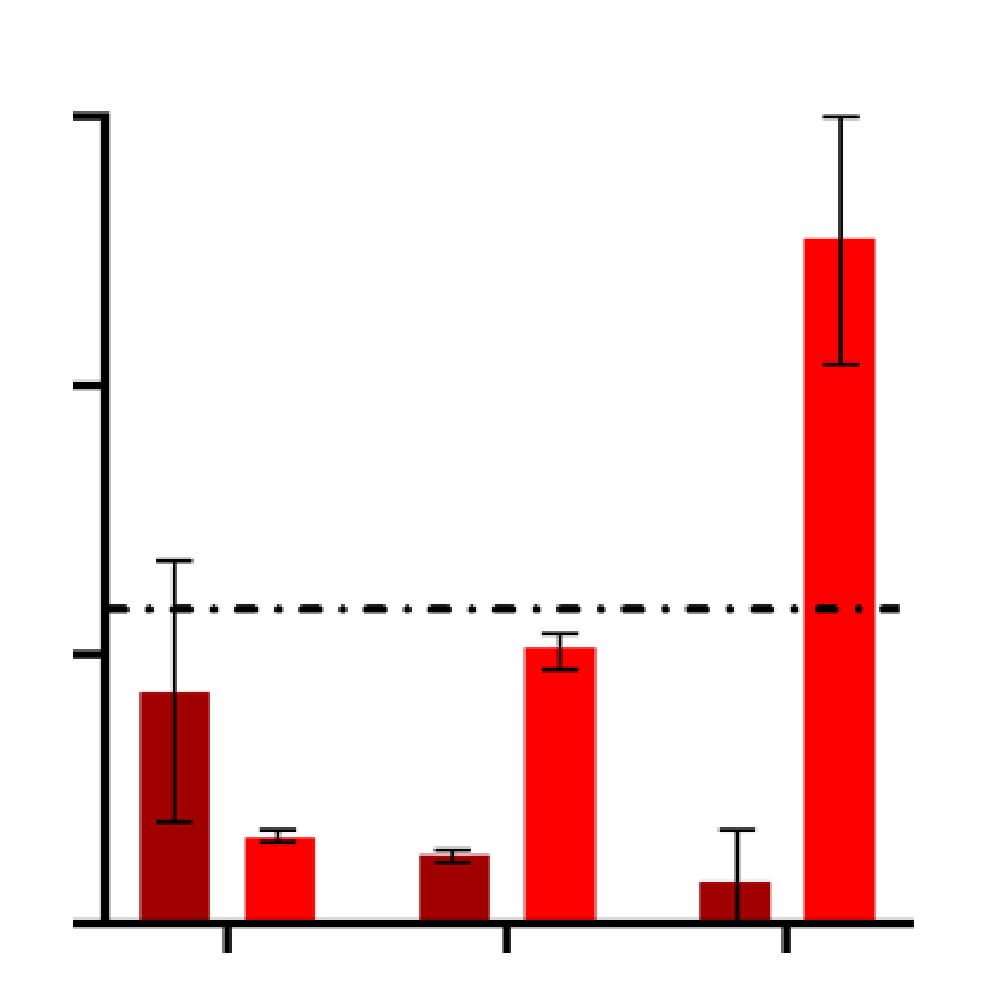
Evaluate and quantify the levels of clinically relevant biomarkers
Assay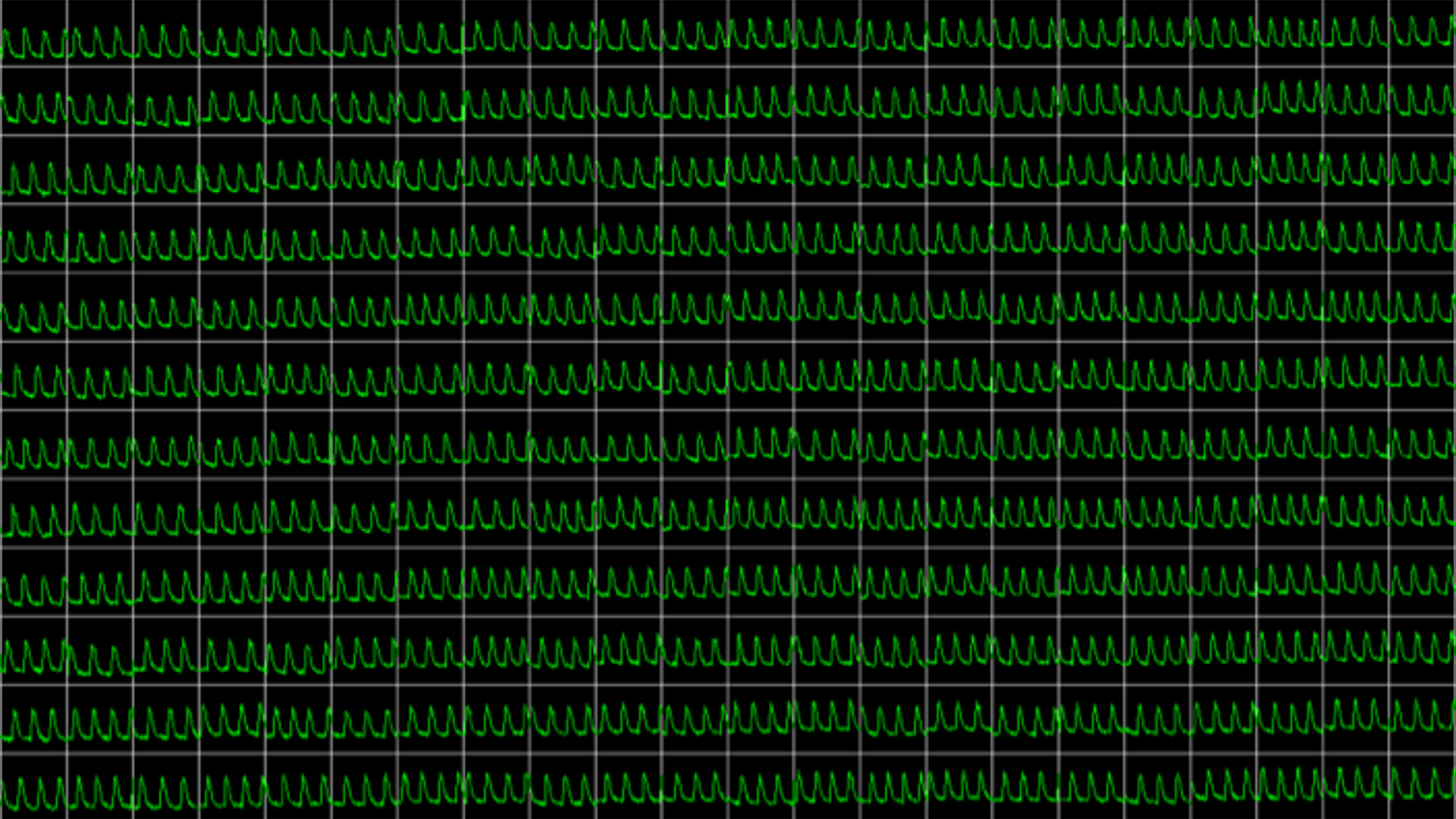
Obtain real-time recordings of intracellular calcium fluctuations
Assay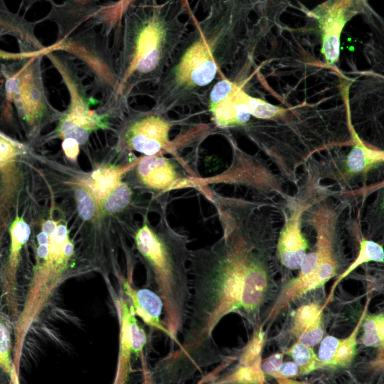
Get in-depth and unbiased insights into the effects of therapeutic candidates on cells
Assay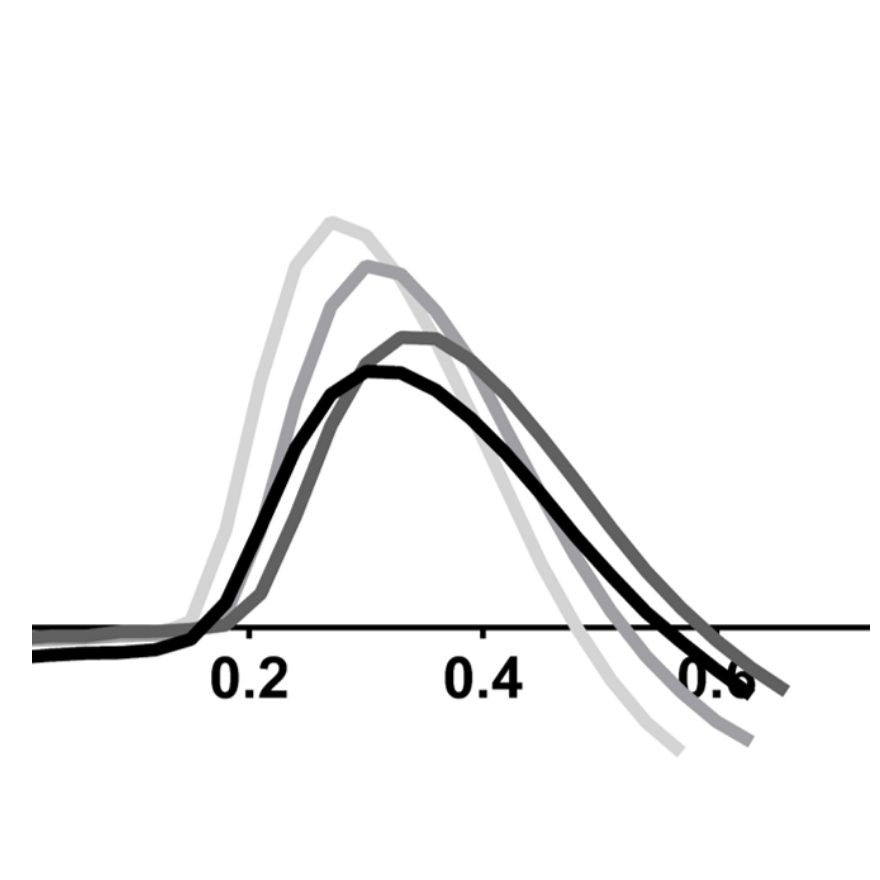
Study drug-induced effects on contractility of cardiac or skeletal muscle cells
Assay
Determine the electrophysiological effects of your therapeutic candidates
Assay
Evaluate endothelial permeability in short and long term
Assay
Study drug-induced phenotypic changes in the cell model of your interest
Assay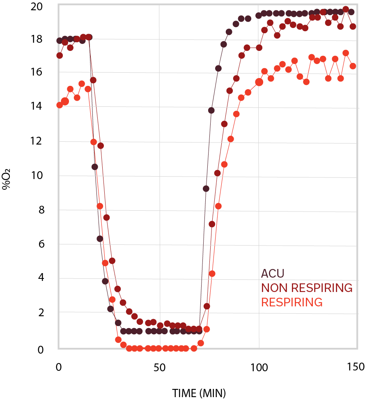
Obtain precise information on compounds' impact on metabolic processes