Metabolism
Metabolism is the sum of biochemical processes that either produce or consume energy. Metabolic dysfunction can be the cause as well as the consequence of many human diseases. Ncardia offers a range of assays to monitor several indicators of the metabolic profile on iPSC-derived cell models. This provides an adaptable platform to assess the impact of candidate compounds on cell metabolism with relevant human in vitro models.
- Monitor key indicators of the metabolic profile
- Extensive menu of assays available
- Multiplexing options
Do you want to explore how this assay can help you progress your drug discovery programs?
Applications
Abnormal cellular metabolism is involved in the development of atherosclerosis, cardiomyopathies and neurodegenerative disorders, among other human diseases. Our scientists have developed and optimized several assays to evaluate the most relevant indicators for metabolic performance, including mitochondrial function, glycolytic activity, oxidative stress and more:
- Oxygen Consumption Rate (OCR) assay
Evaluation of the oxidative phosphorylation capabilities of cells as an indicator of the mitochondrial function.
- Extracellular Acidification Rate (ECAR) assay
Evaluation of the glycolytic activity in cells by recording lactate production.
- Intracellular Oxygen Concentration assay
Quantification of real-time cellular oxygenation under hypoxic conditions and upon drug treatment to investigate the interplay between cellular oxygenation and metabolism.
- TMRE, MitoTracker and JC-1 assays
Monitoring of the mitochondrial membrane potential in whole cells as an indicator of mitochondrial function.
- MitoSOX assay
Assessing the superoxide concentration inside the mitochondria as an indicator of reactive oxygen species toxicity.
- CellROX assay
Novel fluorogenic probe to evaluate oxidative stress in live cells.
Pairing OCR and the ECAR metabolic assays for a comprehensive characterization of metabolic activity in Ncardia’s human iPSC-derived cardiomyocytes.
The combination of these two metabolic assays with physiologically relevant models provides a good indication of drug-induced changes on cellular metabolism to determine drug efficacy and safety. Here we tested the effect of two known compounds, oligomycin and carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP).
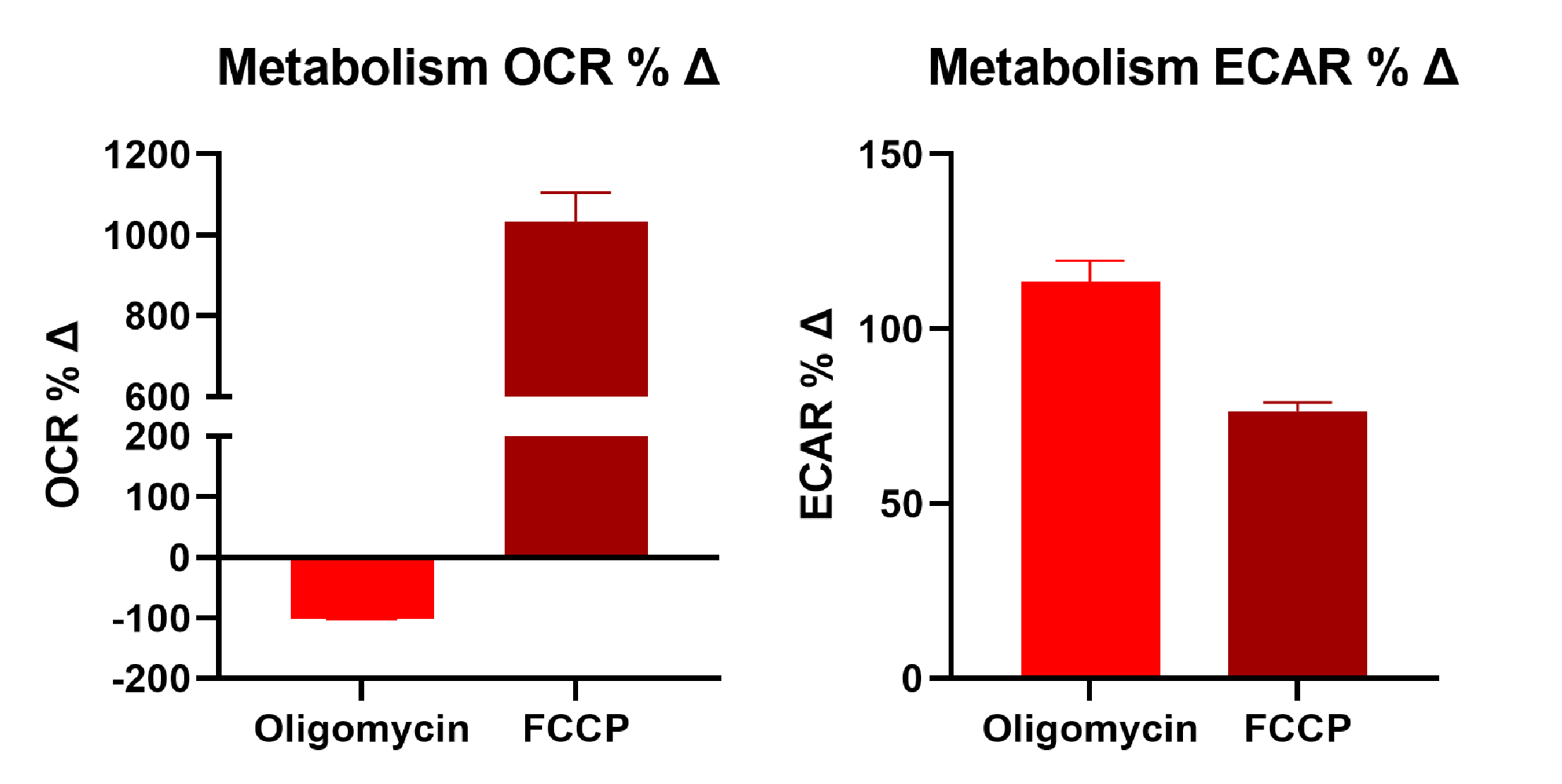 Oligomycin inhibits ATP synthase resulting in reduced mitochondrial respiration (OCR) and increase glycolysis (ECAR). Carbonyl cyanide-4 phenylhydrazone (FCCP) collapses the proton gradient and disrupts mitochondrial membrane potential increasing oxygen consumption (OCR) with no or little effect on glycolysis (ECAR).
Oligomycin inhibits ATP synthase resulting in reduced mitochondrial respiration (OCR) and increase glycolysis (ECAR). Carbonyl cyanide-4 phenylhydrazone (FCCP) collapses the proton gradient and disrupts mitochondrial membrane potential increasing oxygen consumption (OCR) with no or little effect on glycolysis (ECAR).
Assessing mitochondrial depolarization in Ncyte CNS neurons to quantify cytotoxic damage of compounds.
Mitochondrial membrane potential controls respiratory rate, ATP production and generation of reactive oxygen species, which are essential processes for cell homeostasis. Our scientists use confocal microscopy and JC-1 dye as a sensitive readout of mitochondrial depolarization and disruption after treatment with the therapeutic compounds of interest.
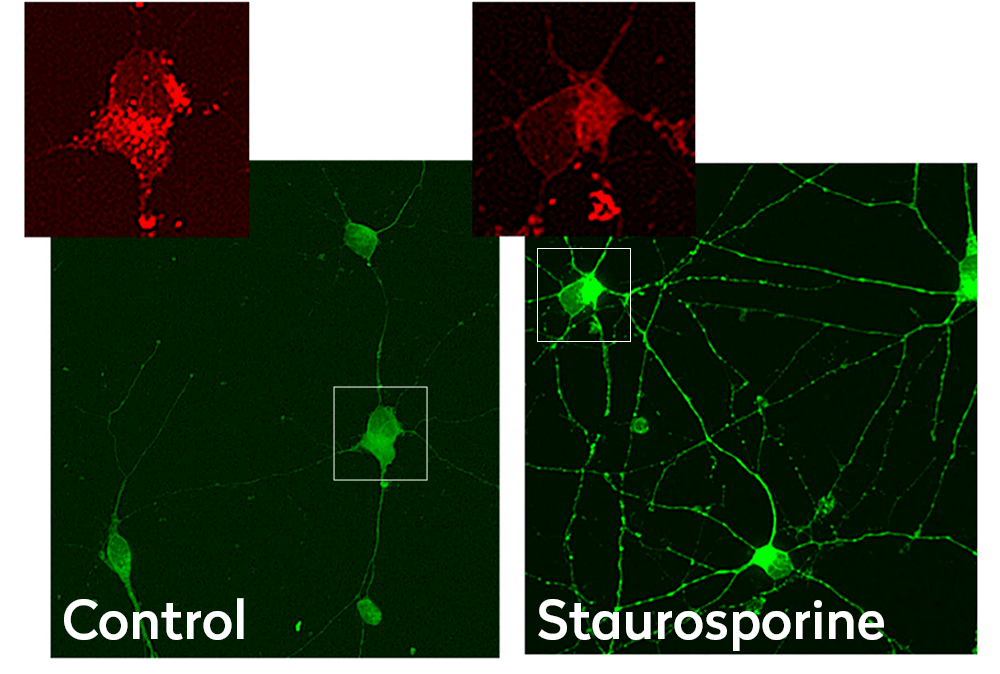 Confocal images of Ncyte CNS neurons stained with JC-1. Emission at 530 nm (green) and 590nm (red) in control and treated cells. Staurosporine treatment reduces mitochondria puncta pattern and depolarization.
Confocal images of Ncyte CNS neurons stained with JC-1. Emission at 530 nm (green) and 590nm (red) in control and treated cells. Staurosporine treatment reduces mitochondria puncta pattern and depolarization.
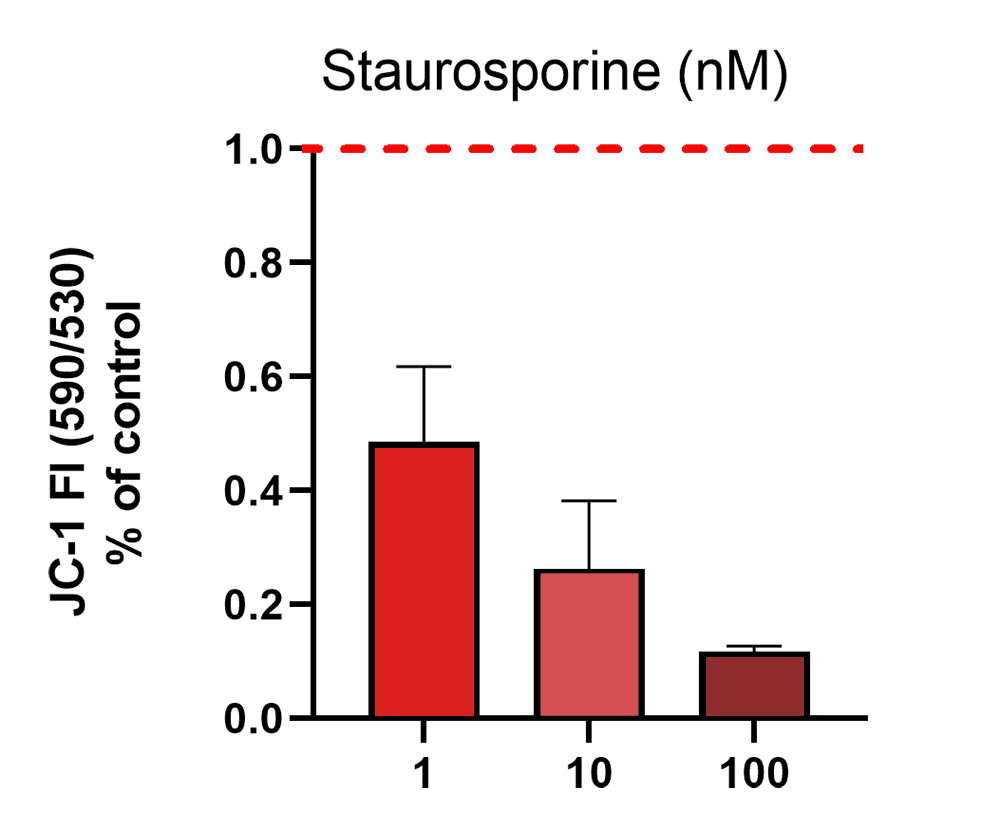 JC-1 590/530 nm emission ratio in Ncyte CNS neurons treated with increasing concentrations of Staurosporine (apoptosis inducer).
JC-1 590/530 nm emission ratio in Ncyte CNS neurons treated with increasing concentrations of Staurosporine (apoptosis inducer).
Assessment of oxidative stress levels in Ncyte Cardiomyocytes to measure changes in cardiac metabolism during ischemia/reperfusion.
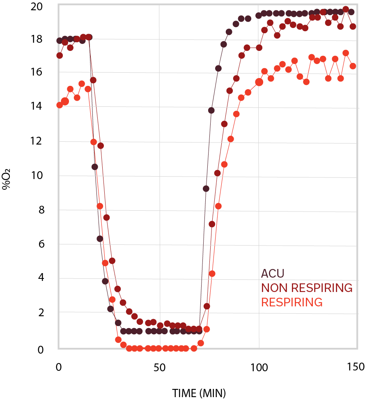
During myocardial infarction, oxygen supply to cardiac tissue is reduced and it is rapidly restored after reperfusion treatment, inducing changes in cell metabolism. Our scientists can model ischemia/reperfusion on human iPSC-derived cardiomyocytes by using an atmospheric control unit (ACU) and measure oxygen consumption in real time to study cells’ metabolism under different environmental conditions.
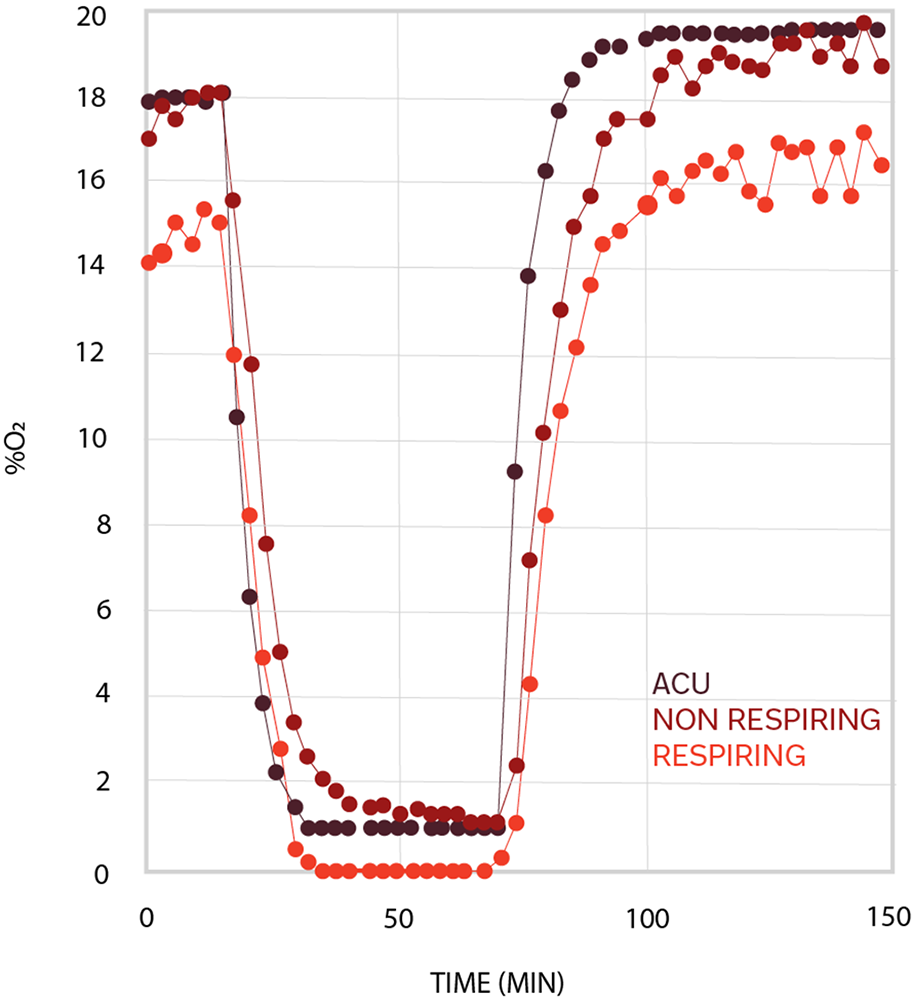 Cell oxygenation traces from cardiomyocytes with oxygen consumption (light red) and from cardiomyocytes in hypoxia (dark red). The black trace represents the oxygen level inside the chamber.
Cell oxygenation traces from cardiomyocytes with oxygen consumption (light red) and from cardiomyocytes in hypoxia (dark red). The black trace represents the oxygen level inside the chamber.
Our work centers on a simple yet powerful premise:
When we combine deep iPSC knowledge, broad assay capabilities and a demonstrated ability to integrate the biology of human diseases into preclinical research, we can help drug developers make critical decisions earlier and with more confidence.
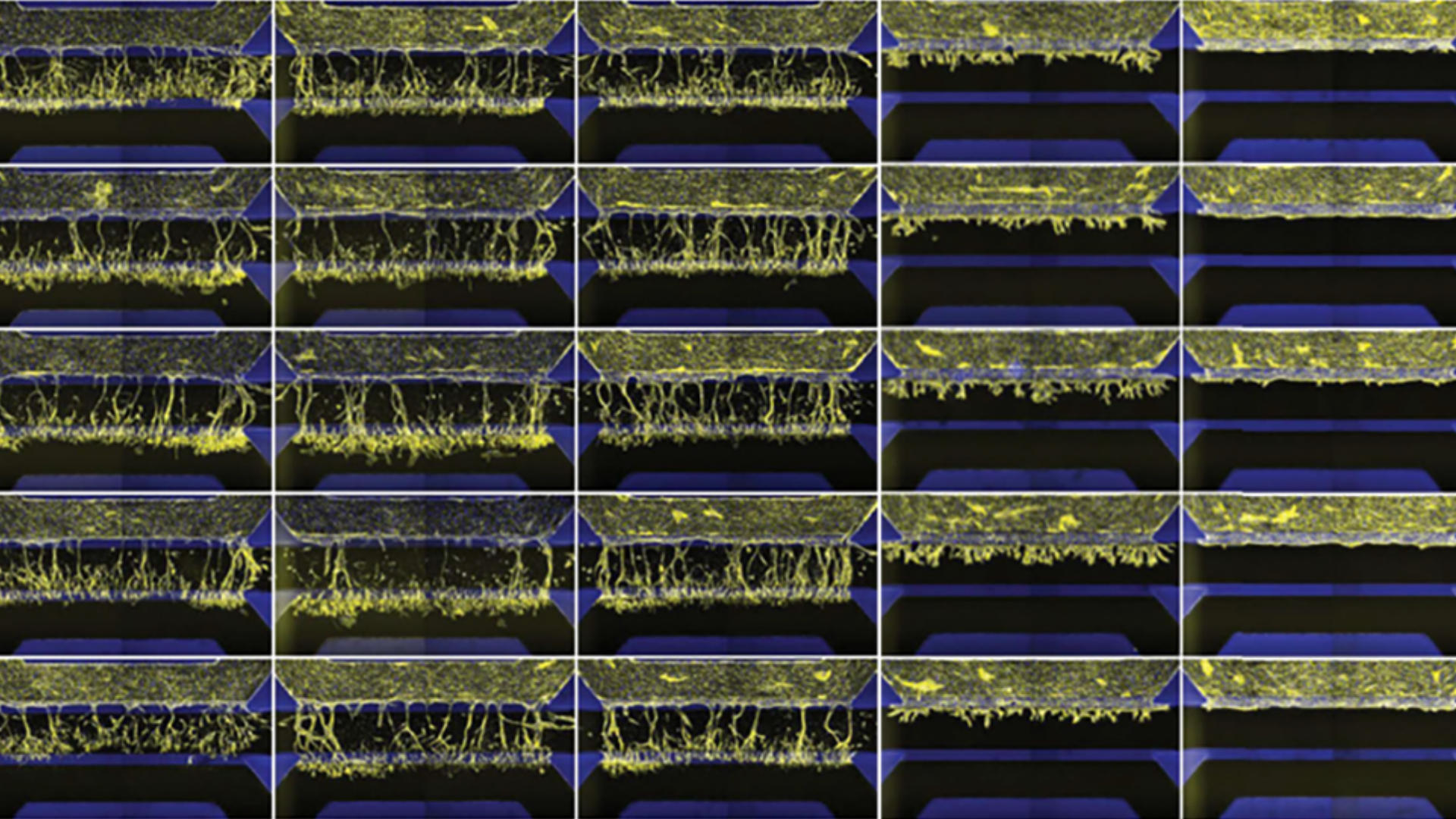
Study compound-induced effects on blood vessel formation
Assay
Evaluate and quantify the levels of clinically relevant biomarkers
Assay
Obtain real-time recordings of intracellular calcium fluctuations
Assay
Get in-depth and unbiased insights into the effects of therapeutic candidates on cells
Assay
Study drug-induced effects on contractility of cardiac or skeletal muscle cells
Assay
Determine the electrophysiological effects of your therapeutic candidates
Assay
Evaluate endothelial permeability in short and long term
Assay
Study drug-induced phenotypic changes in the cell model of your interest
Assay
Obtain precise information on compounds' impact on metabolic processes